Demineralisation
With demineralisation, all salts dissolved in the water are removed through a combination of strongly acidic cation exchangers and strongly basic anion exchangers.
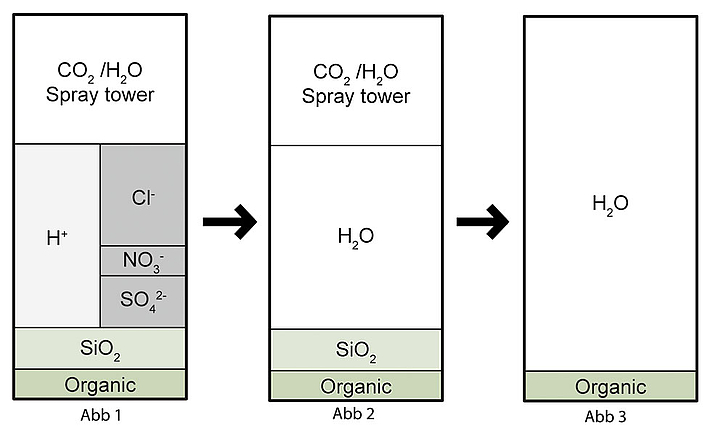
In the first desalination stage, all cations are exchanged for hydrogen ions. The concentrations of anions remain unchanged, i.e. strong acids such as hydrochloric acid HCl, nitric acid HNO3 and sulphuric acid H2SO4, in addition to CO2, arise. This process is also called decationisation for this reason (see Fig. 1).
System technology
Regeneration of cation exchangers primarily occurs with hydrochloric acid.
The acidic discharge from the cation exchanger is passed over an anion exchanger in the second stage. If a weakly basic exchanger is used, only the mineral acids are removed (HCl, H2SO4, HNO3) (see Fig. 2).
If, on the other hand, a strongly basic exchanger is used, silicic acid can also be removed. The result is demineralised water (see Fig. 3).
The regeneration of these exchangers occurs with strong bases, e.g. sodium hydroxide.